Comparing the Best Nitrogen Sources for Plants
The best source of nitrogen for plants should be from? Organic or inorganic?
It depends on the priority that we have to do. For a better environment, of course, the best nitrogen sources for plants is organic.
However, sometimes we are not satisfied with how organic nitrogen works. Most people are impatient and want to get quick and many results.
Because when compared to inorganic nitrogen sources, plant production from organic sources is still lower.
So, the choice of nitrogen sources switches to inorganic sources. if so, we use it equally, between organic and inorganic. Huh … that’s a cliche. Whatever.
I will not discuss how to save the environment. By comparing between organic and inorganic. But, I will discuss the best source of nitrogen for plants.
Beyond organic sources, there are two main sources of nitrogen for plants. Namely, nitrogen in the form of nitrate and nitrogen in the form of ammonium.
Then we will call it nitrate fertilizer and ammonium fertilizer.
Nitrate fertilizer is fertilizer as a source of N (nitrogen) in the form of nitrate ions (NO3-). Although the nitrogen source is not only in the form of nitrate ions. There is ammonium which can also be a source of N for plants.
Whatever the source of nitrogen, nitrogen is a nutrient that is needed by all plants. All types of plants, no exception.
This element is one of the macronutrients which is very important. Especially at the beginning of plant growth or growth of the vegetative phase.
Plants, like vegetables, need nitrogen in all stages of growth. Except fruit vegetables or fruit plants. When the plant is fruiting, the amount of nitrogen should be reduced.
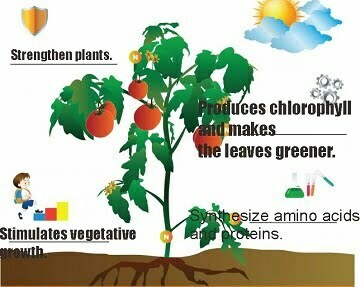
Therefore, nitrogen is very important for plants because of its functions including:
- Stimulates the vegetative growth of plants. Plants grow tall, elongated roots and widened leaves.
- Serves to synthesize amino acids and proteins.
- Strengthen plants.
- To produce green pigments or chlorophyll in leaves.
- Make the leaves more green.
The function of nitrogen above does not depend on what the nitrogen source is. Whether it’s organic nitrogen, nitrate fertilizer, ammonium fertilizer or compound fertilizer, it’s all the same.
As long as it can provide nitrogen to plants, the function of nitrogen can be utilized by plants.
Then, what is the best source of Nitrogen? Nitrate or Ammonium?
So far, I have not found data that states that nitrate fertilizer is better than ammonium fertilizer, or vice versa.
For certain types of plants, the use of nitrogen in the form of nitrates is better.
There are also plants with opposite results. For certain purposes, the use of nitrogen in the form of ammonium can provide more than nitrate fertilizer.
But, the use of nitrate and ammonium together with certain comparisons can also give far more maximum results.
It also depends on the type of land. The use of nitrates in waterlogged fields such as in rice fields is less effective. Conversely, ammonium is more suitable for such land types.
Thus, equalizing that nitrate fertilizer is better than ammonium fertilizer is not a completely correct assumption.
There are still pros and cons to each of these nitrogen sources.
Nitrogen Source from Nitrate vs Ammonium
I have several examples that I can give.
The first is the ratio between nitrate and ammonium in lettuce growth. Lettuce is grown by hydroponics.
Then, the nitrogen source is adjusted so that there is only nitrate or ammonium as its N source.
During 50 days after planting, comparisons of data from hydroponic lettuce growth can be seen in the table below.
| Parameters | Nitrate | Ammonium | 1 Nitrate:1ammonium |
| Root length | 13.6 cm | 16.3 cm | 12.5 cm |
| Root Weight (fresh) | 2.9 grams | 4.9 grams | 2.7 grams |
| Root Weight (dry) | 0.3 grams | 0.5 grams | 0.3 grams |
| Leaf area | 19.1 cm2 | 36.3 cm2 | 19.8 cm2 |
| Stover Weight (fresh) | 4.3 grams | 9.1 grams | 4.2 grams |
| Stover Weight (dry) | 0.8 grams | 2.4 grams | 0.7 grams |
From the hydroponic lettuce data above, we can have a little idea that between nitrogen sources from nitrates or from ammonium there is not so much difference.
Precisely the best growth is lettuce with a source of N nitrate and ammonium. The ratio between nitrate and ammonium in nutrients is 1: 1.
Let’s look at other data so that there are additional comparisons. This time the data was not from lettuce, but on orchid seedlings.
Apparently, the results are a little different and can make us think about this. During the 6 months of planting, we can see the following data from orchid seedling growth:
| Parameter | Nitrate | Ammonium |
| Plant height | 5.95 cm | 5.61 cm |
| Leaf area | 4.25 cm2 | 2.58 cm2 |
| Leaf addition | 4.6 | 3.8 |
At plant height, at first glance, the height of orchid plants is almost the same. But the difference is that nitrates can make orchids higher because of more concentration.
Plant height values above, I take the highest only. For nitrates, the concentration used is 0.5% while ammonium is only 0.4%.
Is when the nitrate concentration is increased, can the orchid’s height be higher?
Apparently not. In the use of more nitrate, plant height is even lower than the 0.5% concentration.
As for the use of ammonium, whatever the concentration used during the study, the height of the orchid plant is almost always equivalent.
From this I see that ammonium is a cheap source of nitrogen for plants. Because with a little concentration, the orchid can grow optimally.
But, nitrates with the right concentration, the potential for vegetative growth of plants can be better. For example, as in the example of orchids above.
The more use of nitrate, it turns out it is not directly proportional to the vegetative development of plants.
That is, the more nitrate is given, not necessarily, the plant will become taller, or wider leaves.
Please look at the table below
Nitrates in orchids with several concentrations of administration | |||
| Accretion | 0.40% | 0.50% | 0.60% |
| Plant height | 3.81 cm | 5.95 cm | 4.00 cm |
| Leaf length | 5.8 | 7.83 | 6.15 |
| Leaf width | 0.6 | 0.6 | 0.55 |
| Leaf area | 3.15 | 4.25 | 3.06 |
| Number of leaves | 3.4 | 4.6 | 3.4 |
| Active root | 6.3 | 12.15 | 6.55 |
The higher the amount of nitrate given does not mean the vegetative growth is also greater. There must be an exact amount. And apparently, this amount is different for each type of plant.
Difference between nitrate and ammonium fertilizer
If you search for the difference between nitrate and ammonium fertilizer, many things can be found.
Both physically and chemically.
Summarizing some of the literature and scientific literature, we can see it in the following points.
1 . Addition of nitrogen in the form of ammonium can accelerate the process of germination of Cattleya labiata, while the source of nitrogen from nitrates actually inhibits germination.
2 . In leaf vegetables, ammonium is more effective in increasing the greenness of the leaves. But excessive ammonium, can make vegetables dry weight dropped dramatically. In other words, plants will contain high water content.
3 . Some nitrate will be used by the roots and some will be directly transported to the stems of plants. Nitrates make plants have higher carbohydrate and carboxylate contents.
4 . Ammonium is more volatile in the form of ammonia gas, whereas nitrate is not. The use of ammonium should be planted into the soil, so that nitrogen is not much wasted through evaporation.
5 . Nitrate is not good for waterlogged agricultural land. Like in rice planting. Rice planting is better to use ammonium nitrogen fertilizer.
6 . Nitrates can be directly utilized by plants. While ammonium must go through a process of degradation that is assisted by bacteria. In other words, nitrate can give nitrogen faster than ammonium.
7 . The difference between nitrate and ammonium fertilizer that is quite important is the price. The price of nitrate fertilizer is much more expensive than ammonium fertilizer.
Nitrogen sources for plant fertilizer
Nitrogen is the most abundant element in nature. But the most amount is in the form of nitrogen gas.
Gas means being in the air or atmosphere. For in the soil, the amount of nitrogen that can be directly absorbed by plants is very small.
Therefore, fertilization to provide N elements for plants is always needed.
These are the sources of N nutrients we can use.
1 Compost
Compost is fertilizer that comes from animal manure or other organic substances such as leaves and organic waste.
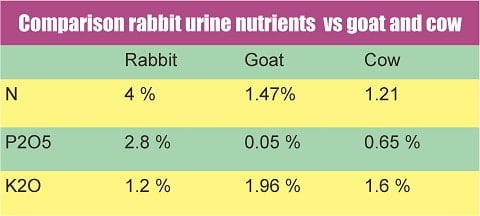
From cow dung, goats, rabbits, chickens, and even human feces are now widely used.
These are all sources of organic N.
However, the amount of N elements present in compost is very small when compared to inorganic fertilizers. Thus, the amount of compost given should be far more. It requires at least 10 times the amount of inorganic fertilizer.
This is an article for making compost from cow and goat manure. There, we will see what percentage of the nitrogen content.
2 Urea
As a source of nitrogen, urea is the most widely used fertilizer today.
This fertilizer just provides nitrogen only. The nitrogen content in urea is as much as 46%.
The chemical formula of urea is CO (NH2) 2. When this fertilizer is sown, ammonia is released and hydrolyzed into ammonium ions.
In the manufacturing process, it combines ammonia gas and carbon dioxide (CO2). But after use, does this CO2 come back into gas again?
If it becomes carbonate, then this will make the soil harden and become stone.
Generally, urea is mixed with the soil or applied to the surface of the soil.
Because of its high solubility (1,080 g / L at 200C), urea can dissolve in water and be applied to the soil in solution, added to irrigation water, or sprayed on plant leaves.
Urea is sprayed on the leaves, quickly absorbed by the leaves.
After urea is sprinkled on the ground, it is quickly hydrolyzed by the urease enzyme into ammonium ions and nitrates. This process naturally takes several days.
3 Ammonium sulfate
Ammonium sulfate consists of ammonium and sulfate. Ammonium as a source of nitrogen and sulfate as a source of sulfur.
The shape is crystal and white with the chemical formula (NH4) 2SO4.
This fertilizer contained nitrogen (N) as much as 21% and sulfur (S) as much as 24%. The total is only 45%, of which 55% are unknown as to what came into the package.
So, for every one sack (50 kg), we add 27.5 kg of material whose function is unclear to our land.
Ammonium sulfate fertilizer is suitable for use in fields with stagnant water. For example rice.
The application of this fertilizer should be buried in the soil. Because if it is only on the surface, nitrogen will evaporate a lot in the form of ammonia gas for non-flooded land.
4 Ammonium nitrate
Ammonium nitrate is a combination of ammonium and nitrate. If we look at the research results above, this fertilizer is very good for plants.
Because from the two data above, giving plants with nitrate and ammonium in almost the same ratio, the results are better.
Its nitrate content makes this fertilizer suitable for cold and hot areas.
Ammonium nitrate fertilizer can burn plants if given too close to the roots or direct contact with leaves.
Availability for plants is very fast, so the frequency of administration should be more frequent. Ammonium nitrate is hygroscopic so it cannot be stored for too long.
The chemical formula of ammonium nitrate is NH4NO3 with nitrogen content between 33 – 34%.
5 Potassium Nitrate
Potassium nitrate is a combination of Potassium (K) and nitrate ions (NO3-).
Nitrate fertilizer is expensive. Because both of them are macronutrients and dissolve well in water.
Potassium is free of chlorine (CL). Usually, this fertilizer is used as a hydroponic fertilizer.
This fertilizer contains more potassium. Potassium is available in the form of K2O oxide compounds. The amount of potassium in this fertilizer is around 44 – 46%. While nitrogen is only 13%.
All available N can be immediately absorbed by plants as nitrate, without the need for other microbial transformation in the soil.
Vegetable and fruit farmers usually prefer to use nutrients with nitrate-based ingredients to improve yields and quality.
Potassium nitrate contains a high amount of K with a ratio of N to K around 1: 3.
Many plants need high K and absorb more K than N at harvest.
6 Calcium Nitrate
This fertilizer is granular, white, dissolves very quickly in water, and is an excellent source of calcium because it contains 19% calcium, Ca.
Other properties are alkaline and hygroscopic reactions.
Just like potassium nitrate, this also includes expensive fertilizer.
There are various brands that we can get on the market.
Reference
[1] Luluk Mukaromah, Tutik Nurhidayati, and Siti Nurfadilah. Effect of Nitrogen Source and Concentration on Growth and Development of Dendrobium laxiflorum J.J Smith Seeds in Vitro. Pomits Science and Arts Journal Vol. 2, No.1, (2013).
[2] Ganda Darmono Nainggolan, Suwardi, and Darmawan. Pattern of Nitrogen Release from Slow Available Fertilizers (Urea-Zeolite-Humic Acid SLOW Release Fertilizer). Department of Soil and Land Resources, Bogor Agricultural University Faculty of Agriculture. Zeolit Indonesia Journal Vol 8 No. 2. November 2009.
[3] Widiastoety, D. Effect of KNO3 and (NH4) 2 SO4 on the Growth of Vanda Orchid Seeds. J. Hort. 18 (3): 30-3-311, 2007.
[4] Hasiholan S., B., Suprihati, Muryas R. Isjwara. Growth and Yield of Lettuce (Lactusa sativa L.) Cultivated Hydroponically. SWCU Agriculture Faculty. Proc. National Seminar on Horticultural Technology Development Entering New Indonesia.
[5] 4T Plant Nutrition – (Right source, Right dose, Right time and Right place) Guidelines for Improving Plant Nutrition Management. International Plant Nutrition Institute Southeast Asia Program.
 JOYNIM FARM Goat Farming, Cattle Farm, Laying Hens, Quail Farm, Gardening
JOYNIM FARM Goat Farming, Cattle Farm, Laying Hens, Quail Farm, Gardening